New Synthetic Pathways to Reactive and Responsive Polymeric Building Blocks

The development of multifunctional nano-materials from the controlled assembly of responsive (block) copolymers requires a high level of control over the molecular structure of the building blocks. In order to transfer a specific functionality from the individual responsive groups to the final material, tailormade block copolymers with complex architectures must be developed.
However, the preparation of such multifunctional block copolymers represents a challenge to polymer chemistry since it often requires the combination of different polymerization methods. These not only have to give access to well defined polymeric architectures but also have to be compatible with the respective functional groups that are implemented to provide the materials final properties. To address this challenge, we are focusing on the development of new robust synthetic pathways by transferring synthetic concepts from modern organic chemistry to the field of polymer synthesis. As shown in the Figure, this interdisciplinary approach gives access to multifunctional polymers and block copolymers of complex architectures.
In the Klinger Lab, we especially concentrate on the development of new reactive precursor polymers that reveal the responsive moieties via a post-polymerization approach. The main challenge in this concept is the development of orthogonal reactivities that enable multiple functionalizations in specific locations.
Selected Publications
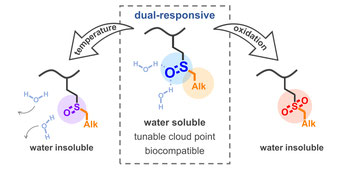
[08] D. Işık, E. Quaas, D. Klinger
Thermo-and oxidation-sensitive poly(meth)acrylates based on alkyl sulfoxides:
dual-responsive homopolymers from one functional group
Polym. Chem. 2020, DOI: 10.1039/D0PY01321H
- featured in the themed collection: Pioneering Investigators

[07] A. Gruber, L. Navarro, D. Klinger
Reactive Precursor Particles as Synthetic Platform for the Generation of
Functional Nanoparticles, Nanogels, and Microgels
Adv. Mater. Interf. 2020, 7, 1901676
Review Article

[06] A. Gruber, D. Işık, B. B. Fontanezi, C. Böttcher, M. Schäfer-Korting, D. Klinger
A versatile synthetic platform for amphiphilic nanogels with tunable hydrophobicity
- featured on the cover of the respective issue

[05] N. V. Handa, S. Li, J. A. Gerbec, N. Sumitani, C. J. Hawker, D. Klinger
Fully Aromatic High Performance Thermoset via Sydnone-Alkyne
Cycloaddition

[04] C. Fleischmann, J. Gopez, P. Lundberg, H. Ritter, K. L. Killops, C. J. Hawker, D. Klinger
A Robust Platform for Functional Microgels via Thiol-Ene Chemistry with Reactive
Polyether-Based Nanoparticles

[03] T. Murakami, T. Kawamori, J. D. Gopez, A. J. McGrath, D. Klinger, K. Saito
Synthesis of PEO‐based physical gels with tunable viscoelastic properties

[02] A. Lee, P. Lundberg, D. Klinger, B. F. Lee, C. J. Hawker, N. A. Lynd
Physiologically Relevant, pH-Responsive PEG-Based Block and Statistical Copolymers with N,N-Diisopropylamine Units

[01] D. Klinger, K. Nilles, P. Théato
Polymeric 1-Iminopyridinium Ylides as New Photo-Switchable Polymers
