Adaptive Micro- and Nanogels for Responsive Interfacial Interactions

In the Klinger Lab, we design adaptive polymer nanogels as modular, functional colloids that bridge biomedical and materials science applications. Responsive polymer micro- and nanogels (NGs) combine colloidal size, structural stability, and highly flexible internal networks. Their structure and chemical functionality can be tailored to control physical properties, diffusion of loaded compounds, and interfacial behavior. This adaptability enables applications ranging from drug delivery of poorly soluble compounds, overcoming biological barriers such as skin, to the design of catalytic interfaces and tackling urgent medical challenges like antimicrobial resistance.
Our approach begins with a molecular understanding of targeted processes, which we translate into synthetic response mechanisms. Using methods such as precipitation polymerization, (mini)emulsion techniques, microfluidics, and self-assembly, we generate nanogels with precise architectures. For characterization, we employ a broad toolkit including dynamic light scattering (DLS), small-angle X-ray scattering (SAXS), nanoparticle tracking analysis (NTA), electron microscopy (TEM/SEM), and atomic force microscopy (AFM). We work in the following main projects:
1. A New Synthetic Platform for Nanogels with Comparable Colloidal Features
A central challenge in nanogel research is achieving accurate structure–property relationships, since different chemical functionalities often change colloidal features like size or crosslinking density. To address this, we have developed a modular nanogel platform based on reactive precursor particles. This platform allows the systematic introduction of functional and responsive groups after particle synthesis, creating nanogel libraries with comparable colloidal properties but distinct chemistries. In combination with orthogonal surface functionalization, this strategy enables us to design precisely tailored nanogels and systematically probe their structure–function relationships. Careful analysis of size distribution, crosslinking density, and surface charge ensures comparability across particle libraries.
2. Amphiphilic Nanogels
Conventional nanogels are limited to hydrophilic networks, whereas our amphiphilic nanogels (ANGs) combine hydrophilic and hydrophobic domains within a single crosslinked structure. Hydrophilic segments provide aqueous swelling and flexibility, while hydrophobic moieties form internal domains that define mechanical properties, influence swelling, and can act as reservoirs for poorly soluble drugs, catalytic species, or substrates. This amphiphilic network architecture enables precise control over diffusion and release of encapsulated compounds through adjustable interactions with the hydrophobic domains.
Amphiphilic Nanogels for Adressing Biological Interfaces and Barriers
In biomedical contexts, ANGs’ dynamic surface amphiphilicity allows them to adapt to complex biological environments such as skin or oral mucosa, facilitating transport across otherwise restrictive barriers. Their amphiphilic networks support mucopenetration, epithelial uptake, and targeted local release of therapeutics. For instance, protease-responsive ANGs enable controlled release of pro-resolving mediators in periodontitis, while optimized internal hydrophobic domains improve intravenous formulations of poorly soluble drugs. We systematically probe these features using calorimetric analysis, solubility parameter calculations, and in vitro barrier models to correlate network structure with therapeutic performance.
Amphiphilic Nanogels for Liquid Interfaces in Materials Science
Beyond biological systems, ANGs self-assemble at liquid–liquid and liquid–air interfaces, where their amphiphilicity and softness govern adsorption, deformation, and interparticle dynamics. This enables the design of particle-stabilized emulsions, foams, and high internal phase emulsions (HIPEs) with tunable stability—important for Pickering emulsions and functional porous materials. Stimuli-responsive modulation of amphiphilicity further allows switching between emulsion types or altering interfacial structures on demand. We use SAXS, interfacial rheology, and advanced microscopy to dissect the mechanisms of interfacial assembly and link them to network design.
3. Stimuli-Responsive Nanogels for Drug Delivery
Stimuli-responsive nanogels represent a versatile platform for next-generation drug delivery. By identifying biological triggers—such as enzymes, pH changes, or redox gradients—and translating them into molecular response mechanisms, we design nanogels that release their cargo in a controlled and targeted fashion.
These functions are integrated into polymeric building blocks and colloidal systems, enabling nanocarriers that dynamically adapt to their environment. Combined with our modular nanogel libraries, this approach offers predictive structure–property insights and supports the rational design of therapeutic nanomaterials. We complement synthesis with systematic biophysical studies of drug loading, release kinetics, and interactions with biological interfaces to optimize therapeutic performance.
Selected Publications

[15] R. Cui, M. Ickler, A. Markovina, S. Kanwal, N. Vogel, D. Klinger*
Amphiphilic Nanogels as Versatile Stabilizers for Pickering Emulsions

[14] H. E. Stauber, C. López-Iglesias, S. Kanwal, E. Quaas, D. Klinger*
Multi-Responsive Nanogels Based on Sulfoxide Polymethacrylates for Biomedical Applications
J.
Polym. Sci., 2025, 63 (7), 1671

[13] C. López-Iglesias, A. Markovina, N. Nirmalananthan-Budau, U. Resch-Genger*, D. Klinger*
Optically Monitoring the Microenvironment of a Hydrophobic Cargo in Amphiphilic Nanogels:
Influence of Network Composition on Loading and Release

[12] C. Biglione, T. M. P. Neumann-Tran, S. Kanwal, D. Klinger*
Amphiphilic micro- and nanogels: Combining properties from internal hydrogel networks, solid
particles, and micellar aggregates
Review Article
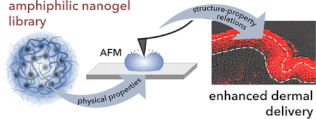
[11] A. Gruber, A. A. Joshi, P. Graff, J. L. Cuéllar- Camacho, S. Hedtrich, D. Klinger*
Influence of Nanogel Amphiphilicity on Dermal Delivery: Balancing Surface Hydrophobicity and Network Rigidity

[10] D. Işık, A. A. Joshi, X. Guo, F. Rancan, A. Klossek, A. Vogt, E. Rühl, S. Hedtrich, D. Klinger
Sulfoxide-functionalized nanogels inspired by the skin penetration properties of DMSO

[09] A. Thünemann, A. Gruber, D. Klinger
Amphiphilic Nanogels: Fuzzy Spheres with a Pseudo-Periodic Internal Structure

[08] T. Bewersdorff, A. Gruber, M. Eravci, M. Dumbani, D. Klinger, A. Haase
Amphiphilic nanogels: influence of surface hydrophobicity on protein corona,
biocompatibility and cellular uptake

[07] A. Gruber, L. Navarro, D. Klinger
Reactive Precursor Particles as Synthetic Platform for the Generation of
Functional Nanoparticles, Nanogels, and Microgels
Adv. Mater. Interf. 2020, 7, 1901676

[06] A. Gruber, D. Işık, B. B. Fontanezi, C. Böttcher, M. Schäfer-Korting, D. Klinger
A versatile synthetic platform for amphiphilic nanogels with tunable hydrophobicity
- featured on the cover of the respective issue

[05] C. X. Wang, S. Utech, J. D. Gopez, M. F. J. Mabesoone, C. J. Hawker, D. Klinger
Non-Covalent Microgel Particles Containing Functional Payloads: Coacervation
of
PEG-Based Triblocks in Microfluidics

[04] C. Fleischmann, J. Gopez, P. Lundberg, H. Ritter, K. L. Killops, C. J. Hawker, D. Klinger
A Robust Platform for Functional Microgels via Thiol-Ene Chemistry with Reactive
Polyether-Based Nanoparticles

[03] D. Klinger and K. Landfester
Stimuli-Responsive Microgels for the Loading and Release of Functional Compounds:
Fundamental Concepts and Applications
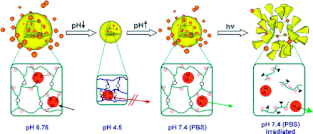
[02] D. Klinger and K. Landfester
Dual Stimuli-Responsive Poly(2-hydroxyethyl methacrylate-co-methacrylic acid) Microgels Based on Photo-Cleavable Cross-Linkers: pH-Dependent Swelling and Light-Induced Degradation

[01] D. Klinger and K. Landfester
Enzymatic- and Light-Degradable Hybrid Nanogels: Crosslinking of Polyacrylamide with Acrylate-Functionalized Dextrans Containing Photocleavable Linkers
